I have acquired full commercial rights to these antibodies, and they are available for licensing through IRBF Development, LLC.
Monoclonal antibodies E1.2D3 and E(mh)1 against CAR (the Coxsackievirus and Adenovirus Receptor)
Monoclonal Antibodies (MoAb) E1.2D3 (also published as MoAb.E1 and MAb.E1) and E(mh)1 (also MAb.E(mh)1) were raised against the recombinant extracellular domain of human CAR. MoAb E1.2D3 reacts with human and rat CAR, but poorly (if at all) with mouse CAR. MoAb E(mh)1 reacts with human and mouse CAR, but poorly (if at all) with rat CAR. Both antibodies have been used successfully for western blots of CAR. Based on the mapping below, MoAb E(mh)1 might well be useful for other applications as well.
Carson, S.D., J.T. Hobbs, S.M. Tracy, and N.M. Chapman. 1999. Expression of the coxsackievirus and adenovirus receptor in cultured human umbilical vein endothelial cells: regulation in response to cell density. J. Virol. 73:7077-7079.
PMID: 10400813 / PMCID: PMC181285
Carson, S.D. 2000. Limited proteolysis of the coxsackievirus and adenovirus receptor (CAR) on HeLa cells exposed to trypsin. FEBS Lett., 484:149-152.
PMID: 11068050
Carson, S.D., B.L. Switzer, S.M. Tracy, and N.M. Chapman. 2004. Monoclonal antibody against mouse CAR following genetic immunization. Hybridoma and Hybridomics 23:19-22.
PMID: 15000844 Full text archived at: Full text
Predicted regions of the CAR protein recognized by the MoAb
The CAR protein is highly conserved among species, and comparison of amino acid sequences (available at www.ncbi.nlm.nih.gov; search CXADR) are informative regarding the most likely regions of the CAR protein recognized by these antibodies.
MoAb E(mh)1 reacts with domain 1 (the amino terminal domain) of human CAR and mouse CAR, but reacts poorly (if at all) with rat CAR. The amino acid sequence alignments shown below reveal two places in domain 1 where rat CAR differs from mouse and human CAR (positions 46 S/E and 131 K/R). The structure diagram places the S/E difference on an exposed loop, and this is a likely position for recognition of human and mouse CAR by the MoAb E(mh)1.
MoAb E1.2D3 reacts with domain 2 (the membrane-proximal domain) of human CAR and rat CAR, but reacts poorly (if at all) with mouse CAR. There are two positions in domain 2 (173 Y/F and 183 K/T) where mouse CAR differs from rat and human CAR. The structure diagram places the K/T difference on an exposed loop, and this is a likely position on human and mouse CAR recognized by MoAb E1.2D3.
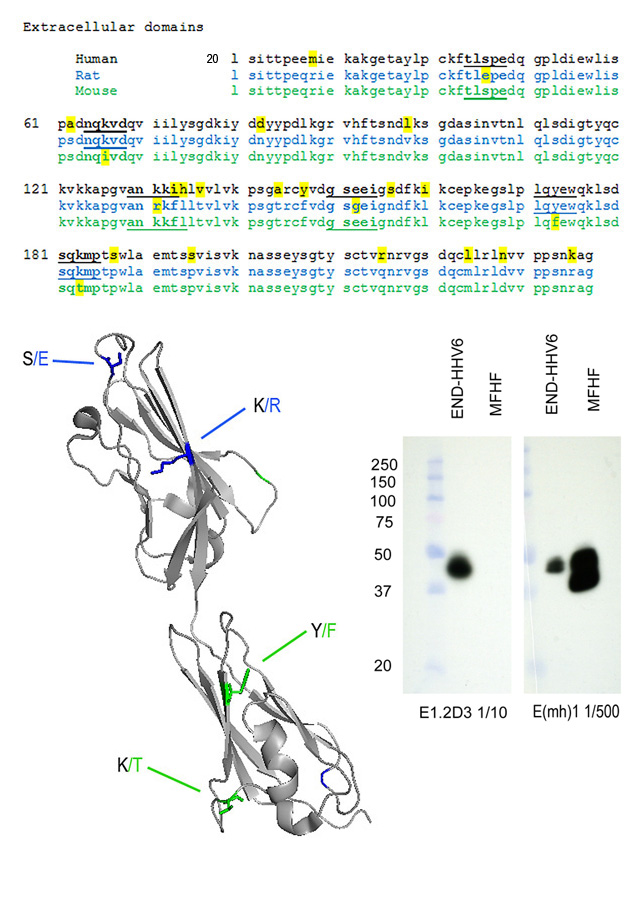
The western blot on the right shows detergent extracts of human END-HHV6 (Luka, J., J. Gubin, C. Afflerback, S.D. Carson, and G.F. Krueger. 1995. Detection of human herpes virus-6 (HHV-6) genomes by in situ PCR in tissue from Kawasaki disease and in coronary arteries of transplanted hearts. Cell Vision-Analyt. Morphol., 2:262-263) and mouse fetal heart fibroblasts (MFHF) probed with the two anti-CAR MoAb. Antibodies were in spent medium diluted as indicated. Bands other than the mature CAR protein near 46k Mr have been reported to be the result of incomplete glycosylation, alternative splicing, or proteolysis. (see Freimuth, P., L. Philipson, and S.D. Carson. 2008. The coxsackievirus and adenovirus receptor (CAR). Current Topics in Microbiology and Immunology 323:67-87. PMID: 18357766).